Comfort, Control, Confidence
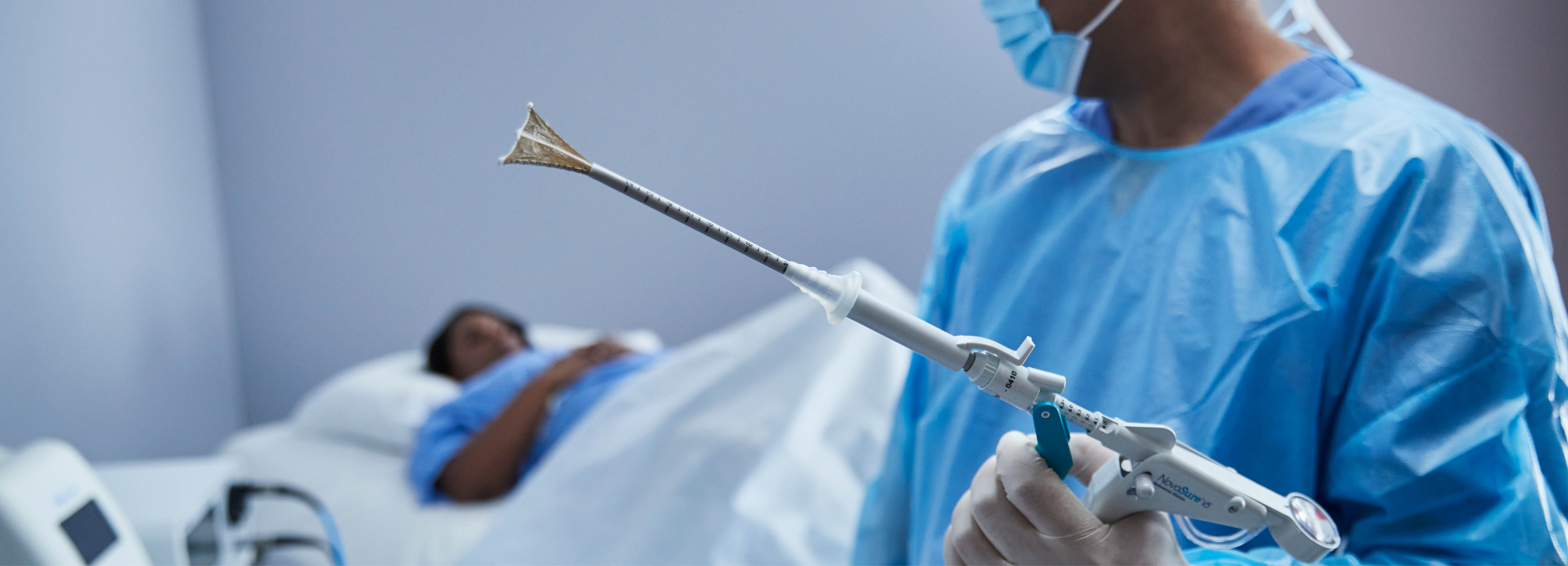
Abnormal uterine bleeding (AUB) can dramatically affect a woman’s comfort, confidence and quality of life. The NovaSure endometrial ablation procedure is a one-time, five-minute,2 minimally invasive procedure, to reduce or stop AUB. The procedure can be conducted in an operating room (OR) and outpatient setting.
Smart-Depth Technology
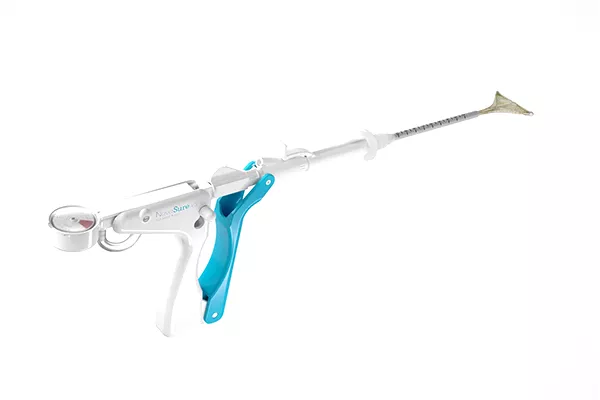
The NovaSure procedure utilizes unique, patented 'Smart-Depth Technology' to deliver customized ablation.
Smart
The technology continuously monitors and measures tissue impedance and calculates the optimal power level required to treat the cavity, based on uterine size.
Unique
Our unique SureClear™ Technology provides constant tissue contact with the array through integrated suction. It simultaneously removes steam, blood, and other by-products.
Safe
The Cavity Integrity Assessment (CIA) is a built-in safety test that confirms uterine cavity integrity, giving you the confidence to perform a safe and effective ablation for every patient.

Improving Patient Care is Within Your Control
For many women, the diagnosis and treatment of uterine disorders is a long, painful and frustrating journey. Our team pioneers gynecological, diagnostic and surgical solutions that give professionals greater confidence in patient diagnosis and more choice over treatment options.
NovaSure Endometrial Ablation is part of the Hologic Uterine Health Portfolio.
Over 20 years of clinical data3
3+ Million
patients treated worldwide4
96%
of women had reduction in bleeding at 5 years5
90%
avoided hysterectomy5
92.8%
patient satisfaction6
Explore the Procedure
Novasure utilizes unique patented technology to deliver customized ablation to each patient. The device is inserted through the cervix to the uterus and emits precisely measured radio-frequency energy to safely remove the endometrium, the uterine lining that sheds during a period.
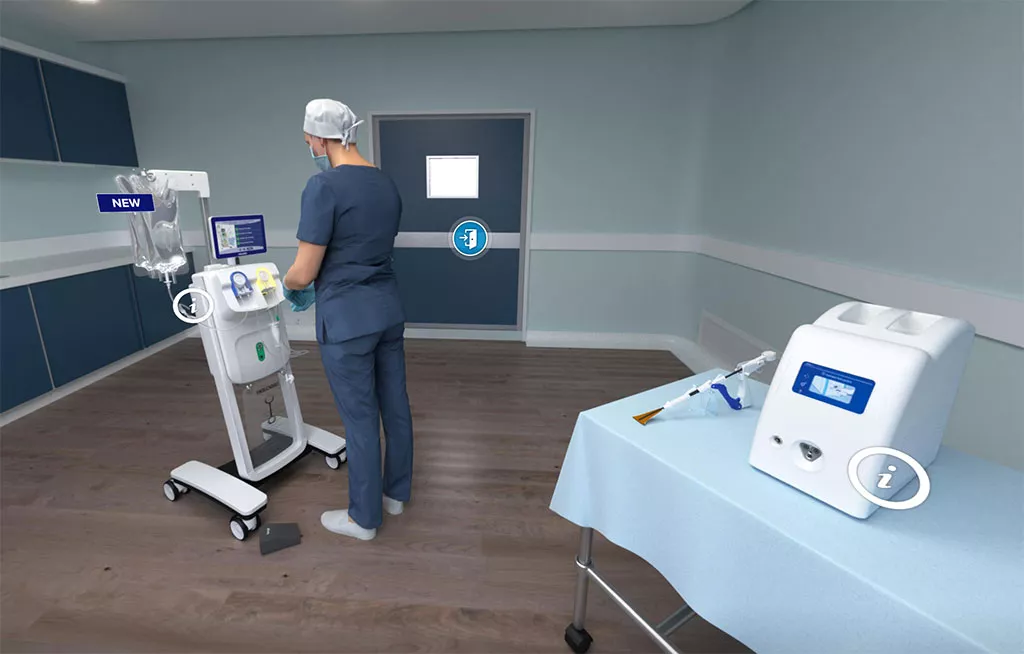
Visit Our Virtual Hospital
See how our Uterine Health products work in an outpatient and operating room (OR) setting.
The NovaSure Endometrial Ablation System
The system consists of a controller and handheld, disposable device. The design has been improved five times since launch, working in close collaboration with customers. However, the core technology has remained the same due to its strong safety record and proven efficacy.
Video Gallery
Evidence. Insight. Collaboration.
Our education portal improves patient care through excellence in education, communication of clinical and scientific evidence, and partnerships with the healthcare community.
Insights
Based on PMA approval in 2001
NovaSure V5 manual MAN-08932-4270, Rev 002
Based on PMA approval in 2001
Hologic, Inc. Data on file; 2004-2018. Based on units shipped from 2004-2018.
Smith PP, Malick S, Clark JT. Bipolar Radiofrequency Compared With Thermal Balloon Ablation in the Office A Randomized Controlled Trial. Obstet Gynecol. 2014 Aug;124 (2 Pt 1):219-25
Cooper J, Gimpelson R, Laberge P, et al. A randomized, multicenter trial of safety and efficacy of the Novasure System in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002; 9:418-428
Documents
Safety Data Sheets
Package Inserts
Frequently Asked Questions
Women with heavy or long-lasting periods who do not wish to have children in the future may be candidates for the NovaSure procedure. If you’re sure you don’t want any children in the future, and more serious causes of heavy bleeding are ruled out, you may be a good candidate for the NovaSure procedure.1
Because NovaSure endometrial ablation treats the lining of the uterus, your chances of getting pregnant after the procedure will be reduced. However, it is still possible to get pregnant if you’re sexually active. A pregnancy after an ablation is very dangerous for both the mother and the fetus, since the uterine lining would not be able to properly support fetal development. It’s very important to discuss what birth control you will use after the NovaSure procedure.1
Immediately after the NovaSure procedure, some women experience some cramping, mild pain, nausea and/or vomiting. Most women feel back to themselves and can resume normal activities within a day or so. Be sure to follow any instructions, no matter how you’re feeling. A watery and/or bloody discharge following the NovaSure procedure is normal. It could start anywhere from immediately after the procedure to a couple of weeks afterwards. The discharge may last only briefly, or for up to a month. It could even come and go, increasing after certain activities. This is quite normal and can be expected with any endometrial ablation procedure.2
Some of the risks associated with the NovaSure endometrial ablation procedure are perforation of the uterus, bleeding, infection, abnormally slow heart beat, injury to organs within the abdomen or around the uterus or complications leading to serious injury or death. These problems are very rare and reported at a rate of less than 0.01%. It’s important to let your doctor know if you have a cardiac pacemaker or any other electrical device in your body.2
Very few patients experience complications following the NovaSure procedure, but you should call your doctor right away if you develop:
- A fever higher than 38°C / 100.4°F.
- Worsening pelvic pain that is not relieved by ibuprofen or other prescribed medicine.
- Nausea, vomiting, shortness of breath, dizziness.
- Bowel or bladder problems.
- A greenish vaginal discharge (reddish, yellowish or brownish is normal).1
When will I know what my periods will be like after the NovaSure procedure?
Every woman is different. Plan to give your body about 3 months to fully heal on the inside and resume its normal cycle. Then, we should be able to tell what your cycle and your periods will be like from that point on.2
The NovaSure system is intended to ablate the endometrial lining of the uterus in pre-menopausal women with menorrhagia due to benign causes for whom childbearing is complete. Pregnancy following the NovaSure procedure can be dangerous.1
What are NovaSure’s contraindications?
The NovaSure procedure should not be offered to a patient with any of the following: A patient who is pregnant or who wants to become pregnant in the future. Pregnancies following ablation can be dangerous for both mother and fetus.
- A patient with known or suspected endometrial carcinoma (uterine cancer) or pre-malignant conditions of the endometrium, such as unresolved adenomatous hyperplasia.
- A patient with any anatomic condition (e.g., history of previous classical cesarean section or transmural myomectomy) or pathologic condition (e.g., long-term medical therapy) that could lead to weakening of the myometrium.
- A patient with active genital or urinary tract infection at the time of the procedure (e.g., cervicitis, vaginitis, endometritis, salpingitis or cystitis).
- A patient with an intrauterine device (IUD) currently in place. Presence of an IUD in the uterine cavity can interfere with a NovaSure procedure.
- A patient with a uterine cavity length less than 4 cm. The minimum length of the electrode array is 4 cm. Treatment of a uterine cavity with a length less than 4 cm will result in thermal injury to the endocervical canal.
- A patient with a uterine cavity width less than 2.5 cm, as determined by the WIDTH dial of the disposable device following device deployment.
- A patient with active pelvic inflammatory disease.1 The NovaSure procedure can be offered to patients with AUB resulting from a variety of conditions including adenomyosis (AUB-A), endometrial dysfunction (AUB-E), underlying coagulopathy (AUB-C), and ovulatory dysfunction (AUB-O).1
In a randomized, multicentre trial, a study found:
- 91% of women returned to normal or light periods, or had no periods at all.
- 41% reported that their periods stopped completely.
- 97% of patients from the initial clinical trial experienced no post-procedural pain.2
Is endometrial ablation protective against endometrial cancer?
The development of endometrial cancer does not seem to be associated with endometrial ablative procedures.2
Based on available data, the need for re-intervention is very low and there is no increased incidence of endometrial cancer or evidence of masking to delay diagnosis in patients who have had an endometrial ablation.2
Hysterectomy rates for NovaSure range from 2-9.8%.2
NovaSure is not contra-indicated to treat patients with multiple previous C-sections and there is no need to scan the myometrial thickness prior to treating patients with NovaSure.1
Both the levonorgestrel-releasing intrauterine system and endometrial ablation strategies lead to a large decrease in menstrual blood loss in women with heavy menstrual bleeding. However, women who start with the levonorgestrel-releasing intrauterine system, a reversible and less invasive treatment, are at an increased risk of needing additional treatment compared with women who start with endometrial ablation.3
Hologic, Inc. Data on file; NovaSure Instructions for Use and Controller Operator’s Manual, MAN-08932-4270 Rev 002.
Cooper J, et al. A randomized, multicentretrial of safety and efficacy of the NovaSure® system in the treatment of menorrhagia .J Am Assoc Gynecol Laparosc. 2002; 9:418-428.
Beelen P, van den Brink MJ, Herman MC, Geomini PMAJ, Dekker JH, Duijnhoven RG, Mak N, van Meurs HS, Coppus SF, van der Steeg JW, Eising HP, Massop-Helmink DS, Klinkert ER, Nieboer TE, Timmermans A, van der Voet LF, Veersema S, Smeets NAC, Schutte JM, van Baal M, Bossuyt PM, Mol BWJ, Berger MY, Bongers MY. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021 Feb;224(2):187.e1-187.e10. doi: 10.1016/j.ajog.2020.08.016. Epub 2020 Aug 12. PMID: 32795428.